Choose the Orbital Diagram for Phosphorus.
It is emphasized that the discussion about the 3d orbital participation in bonding should be based on a reasonable choice of basis sets and it seems suitable to choose the atomic orbitals in proper molecular. Complete the energy-level diagram by filling the number of electrons in each orbital set within a shell and also give the total number of.
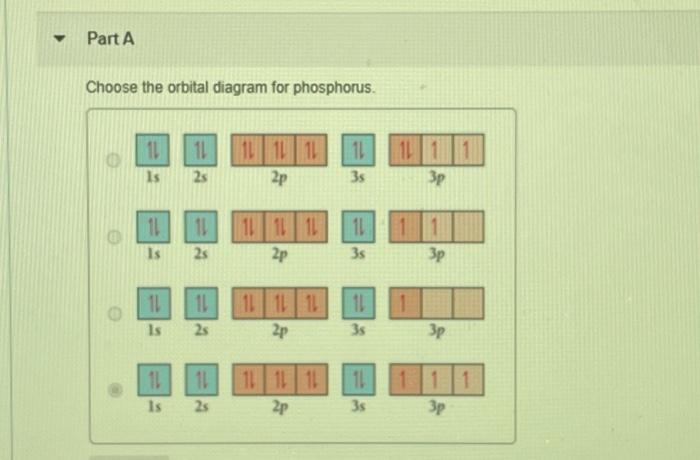
Solved Part A Choose The Orbital Diagram For Phosphorus 11 Chegg Com
Express your answer as an integer.
. A 2p B 1s C 3p D 2s E 3s 4 5 The atomic radius of main-group elements generally increases down a group because _____. Next we go to the to P and were going to fill that up is to p six. Use the buttons at the.
2The electron configuration for phosphorus written in core notation is Ne 3s 3p 3. Therefore the first ten electrons in the electronic. 1s up down 2s up down 2p up down up up Which of the following is the electron configuration of an excited state of an oxygen.
The number of valence electrons impacts on their chemical properties and the specific ordering and properties of the orbitals are important in physics so many students have to get to grips with the basics. These colors ______ has the least energy. The atomic orbital sets of similar energy are grouped into a series of seven shells which is equal to the number of periods in the periodic table.
Phosphorus has atomic number 15. Neon is the noble gas that precedes phosphorus its atomic number is 10. Orbital diagram of Neon Ne 11.
The orbital diagram for germanium is. Orbital diagram of Oxygen O 9. Z15 Number of electrons 15.
Part BBuild the orbital diagram for the ion most likely formed by phosphorus. Because an electron can have either one of two spins any orbital can hold a maximum of four. First thing I would like to do for phosphorus is to ride out its electron configuration.
Orbital diagram of Silicon Si 15. N 3 l 1 ml 101 and ms 1 2 1 2. The next six electrons will go in the 2p orbital.
The atomic number of phosphorus is Write the electron configuration of a phosphorus atom. Orbital diagram of Sodium Na 12. Since phosphorus is in the third group in the P block elements.
1s22s22p63s23p3 is the original electron configuration for PhosphorusIt will gain three electrons leaving it with the same configuration as Ar or 1s22s22p63s23p6. Since 1s can only hold two electrons the next 2 electrons for Phosphorous go in the 2s orbital. The following electronic configurations could be excited states.
What element is represented by this orbital diagram. Electron orbital diagrams and written configurations tell you which orbitals are filled and which are partially filled for any atom. However as explained below probability of transition listed at 2.
In writing the electron configuration for Phosphorus the first two electrons will go in the 1s orbital. The orbital diagram for a ground-state oxygen atom is. The 3p orbital has the following states for electron filling.
So phosphorus is one s two to s to. The aufbau diagram shows the. The p orbital can hold up to six electrons.
The colors of the visible spectrum are blue green orange red violet and yellow. Orbital diagram of Aluminum Al 14. Orbital diagram phosphorus.
Orbital diagram of Magnesium Mg 13. Ii The noble gas configuration of the phosphorus is the next. To do that we need to find the number.
The probability of finding an electron at the nucleus is 0 you will never find an electron in the nucleus. Is more than transition listed at 3. On Orbital Diagram For Germanium.
The lobes of a p orbital disappear at the nucleus. Solution for Examine the orbital diagram for the ground state electron configuration of phosphorus. And then we end at three p three.
Orbital diagram of Phosphorus. Orbital diagram of Fluorine F 10. In the aufbau diagram each box represents an atomic orbital.
11 11 Is 11 2s 101L 1L 2p 11 35 3p 11 11 11 111111 2p Is 2s 3s Зр 10 IL Is LILL 2p 11 3s 25 3p 11 Is 11 25 111111 2p 11 3s 3p Part B Determine the number of unpaired electrons. Choose the correct orbital diagram for the ground state. To write the orbital diagram for the Phosphorus atom P first we need to write the electron configuration for just P.
Electron Configuration Ar 3d10 4s2 4p2. V Vanadium What element is represented by this orbital diagram. What does this tell us about electrons in p orbitals.
What two things does Hunds rule tell us. Then we go to the third period and we Philip the S. Orbital diagram of Nitrogen N 8.
Electrons Per Shell 2 8 18 4. 4 In which orbital does an electron in a phosphorus atom experience the greatest effective nuclear charge. Orbital diagram of Carbon C 7.
Chemistry questions and answers. P Phosphorus What element is represented by this orbital diagram. I The phosphorus with atomic number Z 15 has the following electron configuration.
In writing the electron configuration for Phosphorus the first two electrons will go in the 1s. Ab initio LCAOMOSCF calculations for several typical molecules containing phosphorus have been undertaken to study the role of phosphorus 3d orbitals in the bonding. Part A Choose the orbital diagram for phosphorus.
You can obtain correct electron configurations for the elements up to. Group of answer choices A 0 B 6 C 2 D 3 E 1. A the principal quantum number of the valence orbitals increases.
2 electrons in the. Solution for How many unpaired electrons are in the orbital diagram for phosphorus. 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p2.

How To Write The Orbital Diagram For Phosphorus P Youtube

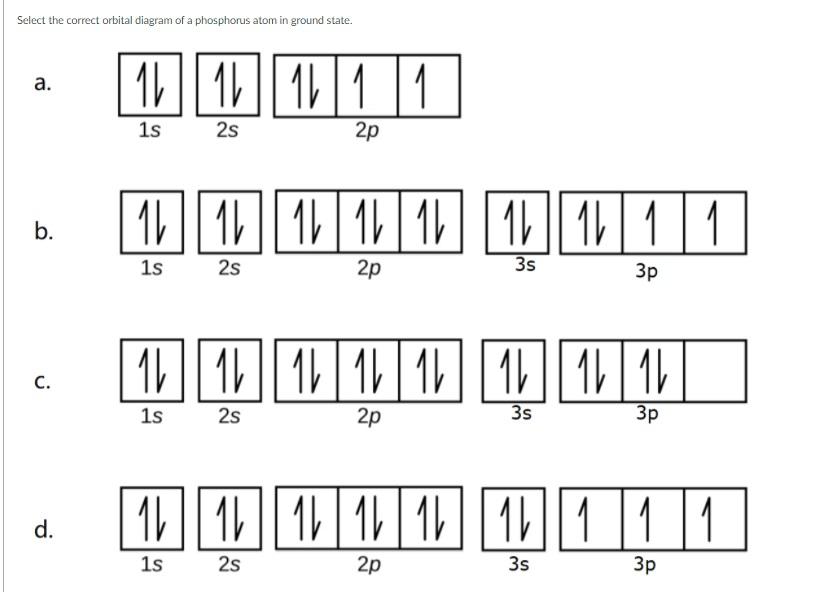
Solved Select The Correct Orbital Diagram Of A Phosphorus Chegg Com

Comments
Post a Comment